
Department of
Food Science and Technology

5 Degree Programs
Food Science and Technology encompasses a unique blend of science, art, technology, design and business. The Texas A&M Department of Food Science and Technology offers three undergraduate and three graduate degrees that prepare students for a myriad of diverse professional opportunities.

20 Research Faculty
Our research strengths include specialized expertise in food science technologies, encompassing food chemistry, engineering, microbiology and processing, with an unique access to a wide array of laboratories and pilot facilities.

100% Employment Rate
The Department of Food Science and Technology takes immense pride in its outstanding track record of a 100% graduate employment rate. This remarkable achievement is a testament to the department’s unwavering commitment to equipping students with the knowledge and skills needed to excel in the dynamic and competitive field of food science.

Why Food Science?
Food Science and Technology is an exciting field that prepares students to develop new food products, design innovative processing technologies, improve food quality and nutritive value, and enhance food safety and health promoting properties.
Our graduates have a competitive edge for positions in food industry, regulatory agencies and graduate school opportunities. They are employed by leading food and beverage companies such as Pepsi Co., General Mills Inc., Kellogg’s, HEB and more.

Why Food Science?
Food Science and Technology is an exciting field that prepares students to develop new food products, design innovative processing technologies, improve food quality and nutritive value, enhance food safety and health promoting properties. Our graduates have a competitive edge for positions in food industry, regulatory agencies and graduate school opportunities. They are employed by leading food and beverage companies such as Pepsi Co., General Mills Inc., Kellogg’s, HEB and more.

Join the Food Science Club
The Food Science Club at Texas A&M University is open to all undergraduate or graduate students who are interested in a career in any aspect of the food industry. The club offers activities that will encourage networking and leadership and educate members on the principles of food science.

Undergraduate Spotlight
Alisha Patterson
Along with her father, a Texas native, Patterson visited the Texas A&M College of Agriculture and Life Sciences Department of Food Science and Technology for a meet-and-greet event on Aggieland Saturday during her junior year of high school.
“Everybody seemed really supportive of the students, like they wanted to help build the students to be their best selves and help them further their career,” Patterson said. “That’s what really attracted me to study food science at Texas A&M.”

Alumni Spotlight
Janet Adams
During her teenage years, Adams developed a growing interest in the health-related aspects of food. After graduating from Winston Churchill High School, she decided to attend Texas A&M University. It was during her second semester at Texas A&M, particularly after taking an Introduction to Food Science class, that she became certain food science was the career path she wanted to pursue.

Faculty Spotlight
Mian Riaz, Ph.D.
When Mian Riaz, Ph.D. steps into a classroom, one of his goals is to help students gain insights into the customs and values of different communities by exploring and celebrating the diversity in foods.
Since joining Texas A&M University more than 30 years ago, Riaz, has established himself not only as an internationally recognized expert in religious and ethnic foods, but also an expert in the extrusion technology that forms processed foods into their desired shapes and sizes.
Department Updates
-

IFANCA endows $300,000 for Dean’s Excellence Scholarship in Food Science and Technology
The IFANCA Endowed Dean’s Excellence Scholarship in Food Science and Technology was established in 2024…
-
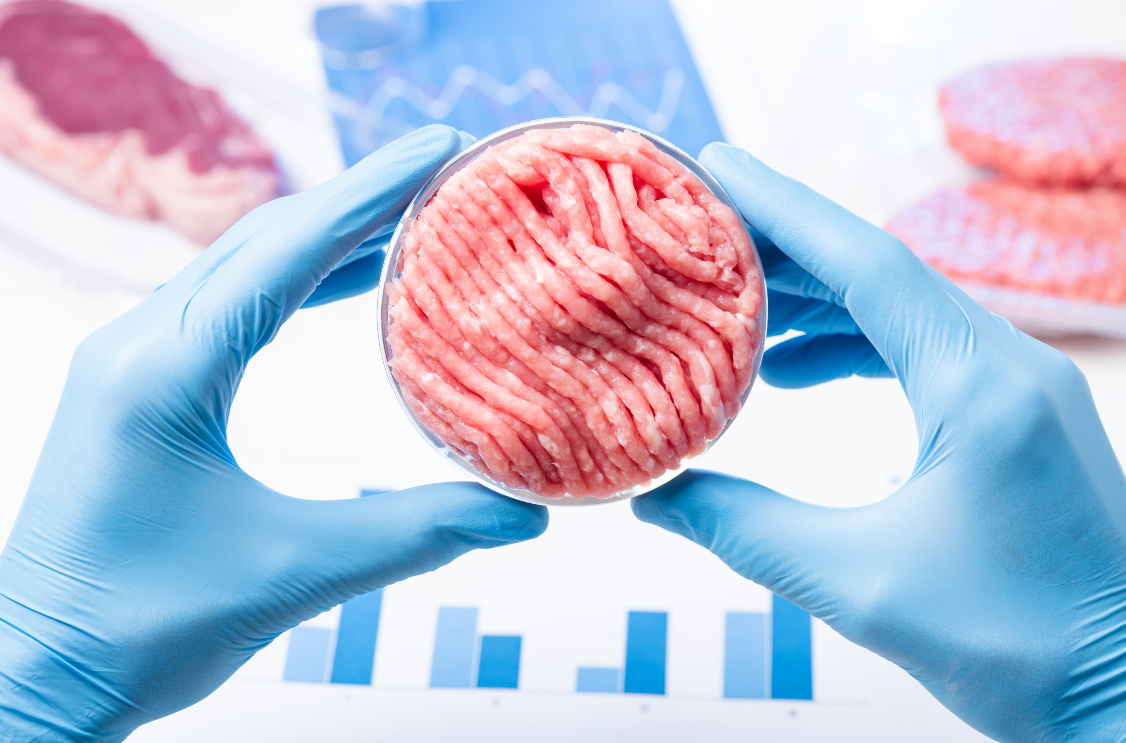
FSTC’s Dr. Ovissipour receives 2.5M grant from USDA-SAS for research on Cellular Agriculture
The demand for meat and animal-based products is increasing dramatically as the world population is…
-

Culinary Creativity Unleashed: Food Science Club Cooking Competition!
The Food Science Club orchestrated a highly triumphant Cooking Contest, drawing in students from Texas…
Food Science and Technology News

Sharing the sensational side of food science
Originally from the suburbs of New Orleans, Alisha Patterson always enjoyed cooking and baking, but she found her first real taste for food science through the National FFA Organization’s Agriscience Fair.

Food technology expert gives Texas A&M students a taste of other cultures
When Mian Riaz, Ph.D. steps into a classroom, one of his goals is to help students gain insights into the customs and values of different communities by exploring and celebrating the diversity in foods.
Have Questions?
For degrees or admissions questions:
For general questions: